May 09 @ 13:00 – 14:00
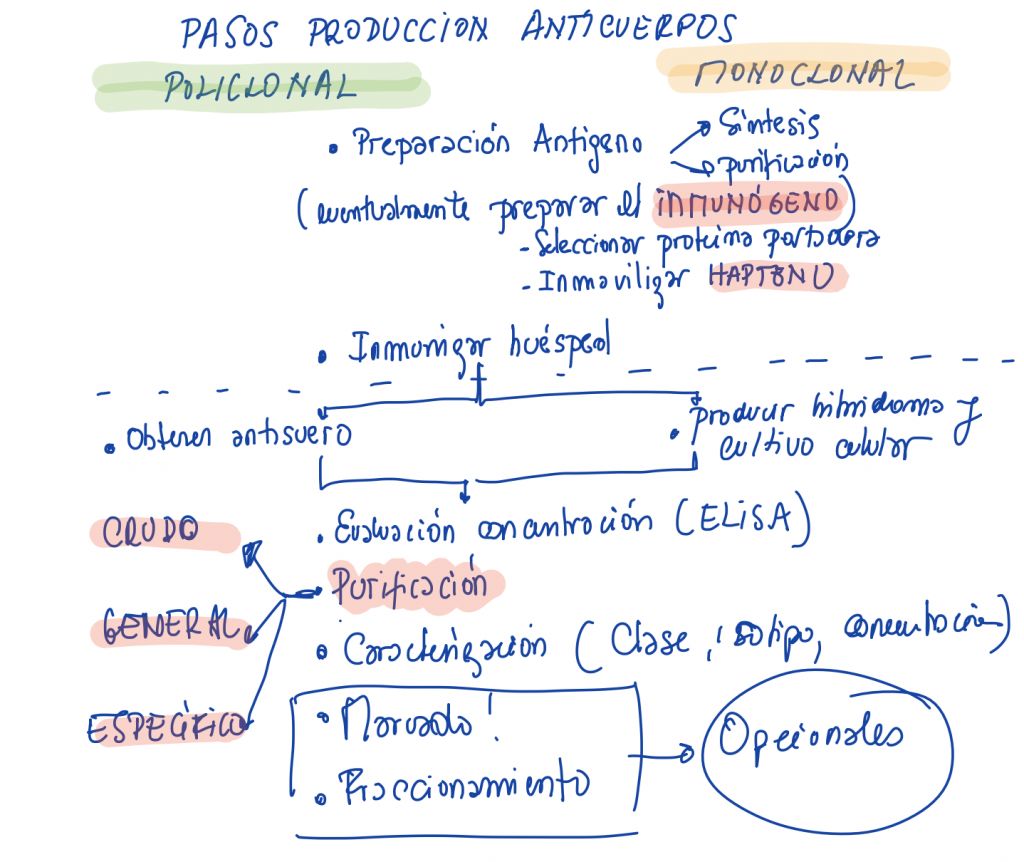
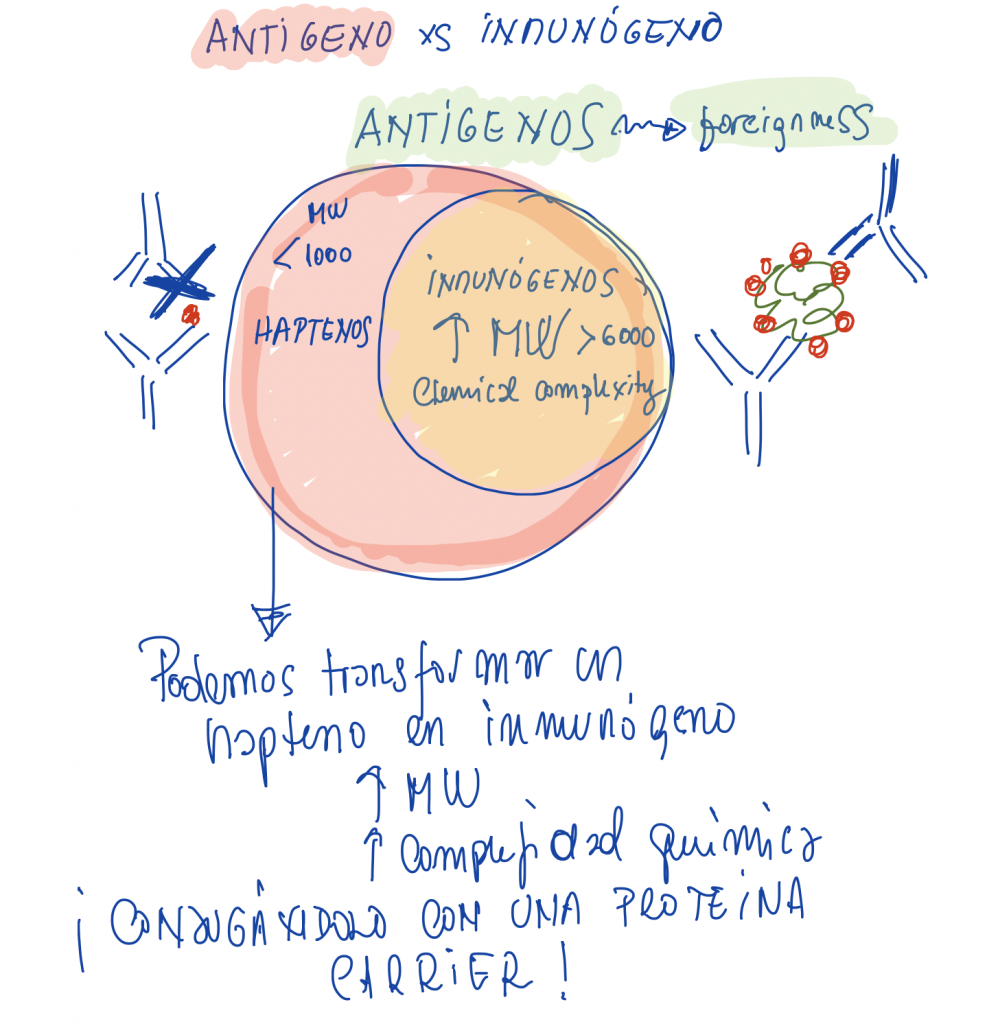
Production of bioreactives and biomolecules. Synthesis of oligonucleotides and peptides in solid phase. Production of monoclonal and polyclonal antibodies. Synthesis of immunogenic haptens.
Instructions
It is highly recommended to revise Lesson 2, related to antibody structure, antibody-antigen interaction and classes and subclasses of immunoglobulins
Lesson 2. Structure of biomolecules and Biorecognition. Amino Acids, Peptides and Proteins. Antibodies. Enzymes. Nucleic Acids. Biorecognition: Enzyme/Substrate. Antigen/antibody. Hybridization. Other affinity interactions in nature. Strept(avidin), Protein A and G. Aptamers. Biomimetic recognition. The importance of water in biorecognition. Biological buffers.
Self-assessment test. Antibody structure
It is highly recommended to pass the test before continue with polyclonal and monoclonal antibodies
BLACKBOARD NOTES
LECTURES
Additional contents
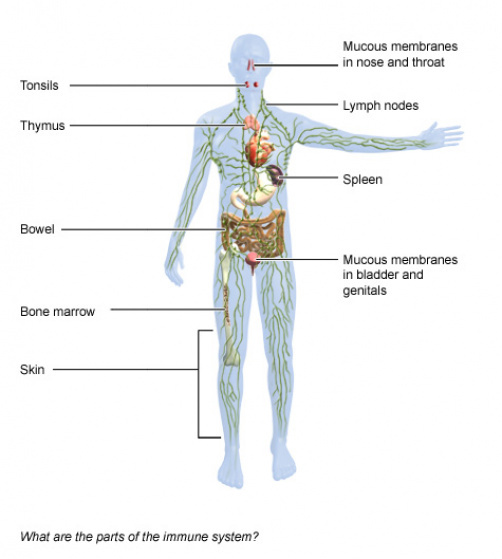
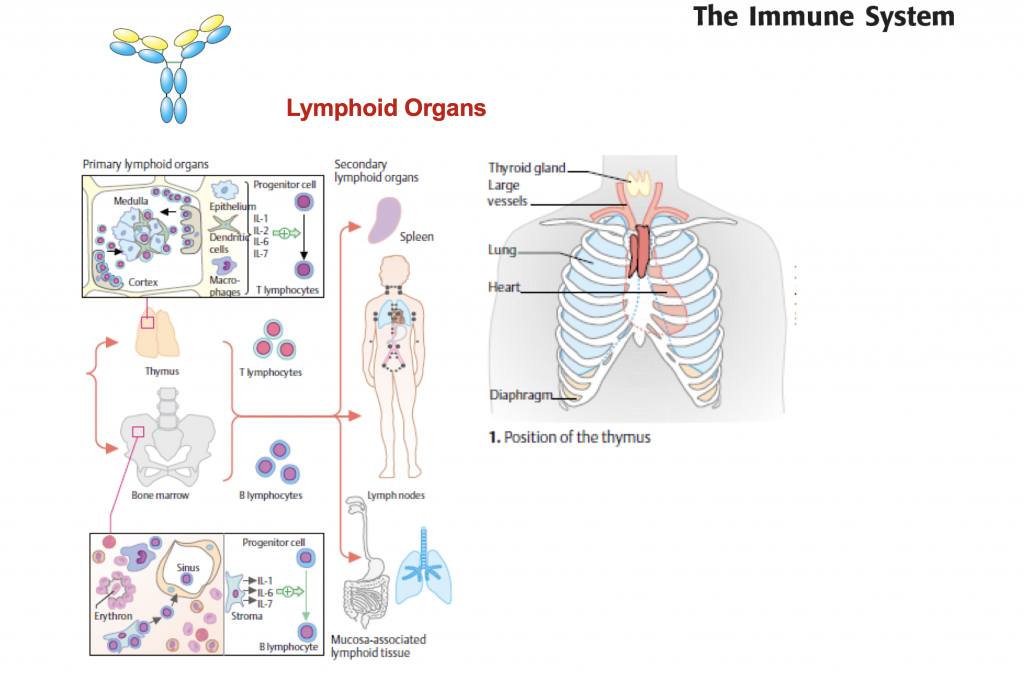
Lymphoid organs
The lymphatic system is composed of:
- Primary lymphoid organs: These organs include the bone marrow and the thymus. They create special immune system cells called lymphocytes.
- Secondary lymphoid organs: These organs include the lymph nodes, the spleen, the tonsils and certain tissue in various mucous membrane layers in the body (for instance in the bowel). It is in these organs where the cells of the immune system do their actual job of fighting off germs and foreign substances.
Bone marrow
Bone marrow is a sponge-like tissue found inside the bones. That is where most immune system cells are produced and then also multiply. These cells move to other organs and tissues through the blood. At birth, many bones contain red bone marrow, which actively creates immune system cells. Over the course of our life, more and more red bone marrow turns into fatty tissue. In adulthood, only a few of our bones still contain red bone marrow, including the ribs, breastbone and the pelvis.
Thymus
The thymus is located behind the breastbone above the heart. This gland-like organ reaches full maturity only in children, and is then slowly transformed to fatty tissue. Special types of immune system cells called thymus cell lymphocytes (T cells) mature in the thymus. Among other tasks, these cells coordinate the processes of the innate and adaptive immune systems. T cells move through the body and constantly monitor the surfaces of all cells for changes.
Lymph nodes
Lymph nodes are small bean-shaped tissues found along the lymphatic vessels. The lymph nodes act as filters. Various immune system cells trap germs in the lymph nodes and activate the creation of special antibodies in the blood. Swollen or painful lymph nodes are a sign that the immune system is active, for example to fight an infection.
Spleen
The spleen is located in the left upper abdomen, beneath the diaphragm, and is responsible for different kinds of jobs:
- It stores various immune system cells. When needed, they move through the blood to other organs. Scavenger cells (phagocytes) in the spleen act as a filter for germs that get into the bloodstream.
- It breaks down red blood cells (erythrocytes).
- It stores and breaks down platelets (thrombocytes), which are responsible for the clotting of blood, among other things.
There is always a lot of blood flowing through the spleen tissue. At the same time this tissue is very soft. In the event of severe injury, for example in an accident, the spleen may rupture easily. Surgery is then usually necessary because otherwise there is a danger of bleeding to death. If the spleen needs to be removed completely, other immune system organs can carry out its roles.
Tonsils
The tonsils are also part of the immune system. Because of their location at the throat and palate, they can stop germs entering the body through the mouth or the nose. The tonsils also contain a lot of white blood cells, which are responsible for killing germs. There are different types of tonsils: palatine tonsils, adenoids and the lingual tonsil. All of these tonsillar structures together are sometimes called Waldeyer’s ring since they form a ring around the opening to the throat from the mouth and nose.
There is also lymphatic tissue on the side of the throat, which can perform the functions of the palatine tonsils if they are removed.
Mucous membranes
The bowel plays a central role in defending the body against germs: More than half of all the body’s cells that produce antibodies are found in the bowel wall, especially in the last part of the small bowel and in the appendix. These cells detect foreign substances, and then mark and destroy them. They also save information about the substances in order to be able to react more quickly the next time. The large bowel also contains harmless bacteria called gastrointestinal or gut flora. Healthy gut flora make it difficult for germs to spread and enter the body.
Mucous membranes support the immune system in other parts of the body, too, such as the respiratory and urinary tracts, and the lining of the vagina. The immune system cells are directly beneath the mucous membranes, where they prevent bacteria and viruses from attaching.Go to:
Sources
- Brandes R, Lang F, Schmidt R (Ed). Physiologie des Menschen: mit Pathophysiologie. Berlin: Springer; 2019.
- Menche N (Ed). Biologie Anatomie Physiologie. München: Urban und Fischer; 2016.
- Pschyrembel. Klinisches Wörterbuch. Berlin: De Gruyter; 2017.
- IQWiG health information is written with the aim of helping people understand the advantages and disadvantages of the main treatment options and health care services.Because IQWiG is a German institute, some of the information provided here is specific to the German health care system.
Classifications of Adaptive Immunity
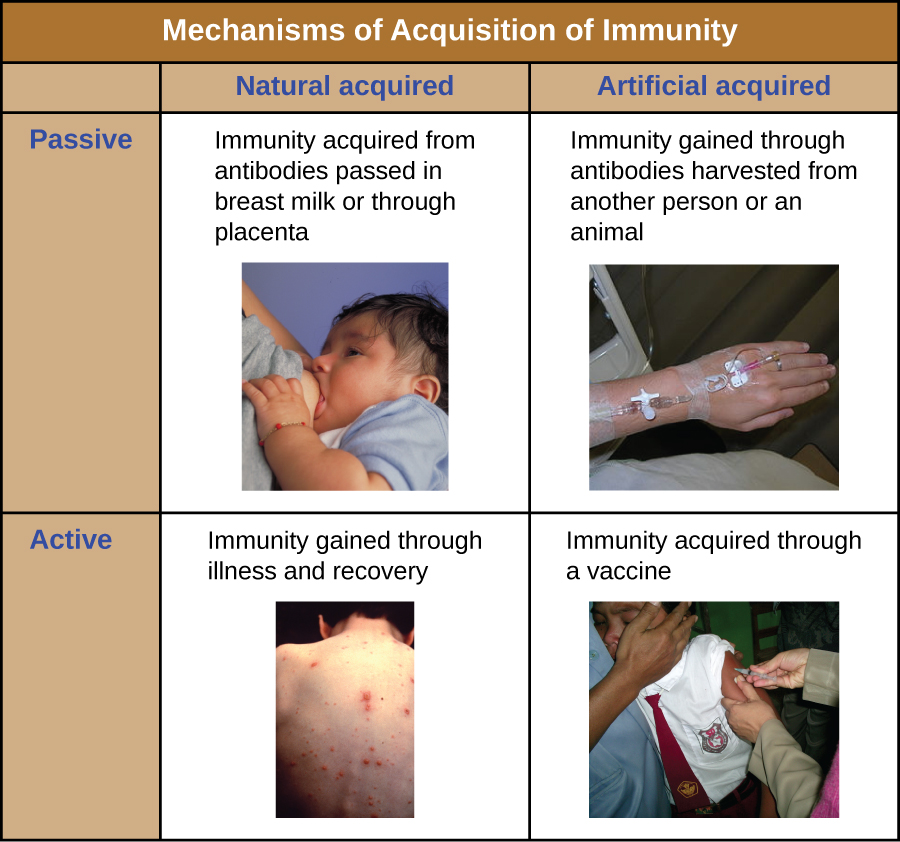
All forms of adaptive immunity can be described as either active or passive.
Active immunity refers to the activation of an individual’s own adaptive immune defenses, whereas passive immunity refers to the transfer of adaptive immune defenses from another individual or animal. Active and passive immunity can be further subdivided based on whether the protection is acquired naturally or artificially.
Natural active immunity is adaptive immunity that develops after natural exposure to a pathogen. Examples would include the lifelong immunity that develops after recovery from a chickenpox or measles infection (although an acute infection is not always necessary to activate adaptive immunity). The length of time that an individual is protected can vary substantially depending upon the pathogen and antigens involved. For example, activation of adaptive immunity by protein spike structures during an intracellular viral infection can activate lifelong immunity, whereas activation by carbohydrate capsule antigens during an extracellular bacterial infection may activate shorter-term immunity.
Natural passive immunity involves the natural passage of antibodies from a mother to her child before and after birth. IgG is the only antibody class that can cross the placenta from mother’s blood to the fetal blood supply. Placental transfer of IgG is an important passive immune defense for the infant, lasting up to six months after birth. Secretory IgA can also be transferred from mother to infant through breast milk.
Artificial passive immunity refers to the transfer of antibodies produced by a donor (human or animal) to another individual. This transfer of antibodies may be done as a prophylactic measure (i.e., to prevent disease after exposure to a pathogen) or as a strategy for treating an active infection. For example, artificial passive immunity is commonly used for post-exposure prophylaxis against rabies, hepatitis A, hepatitis B, and chickenpox (in high risk individuals). Active infections treated by artificial passive immunity include cytomegalovirus infections in immunocompromised patients and Ebola virus infections. In 1995, eight patients in the Democratic Republic of the Congo with active Ebola infections were treated with blood transfusions from patients who were recovering from Ebola. Only one of the eight patients died (a 12.5% mortality rate), which was much lower than the expected 80% mortality rate for Ebola in untreated patients.2 Artificial passive immunity is also used for the treatment of diseases caused by bacterial toxins, including tetanus, botulism, and diphtheria.
Artificial active immunity is the foundation for vaccination. It involves the activation of adaptive immunity through the deliberate exposure of an individual to weakened or inactivated pathogens, or preparations consisting of key pathogen antigens.
Classes of Vaccines included in the calendar (classical vaccines)
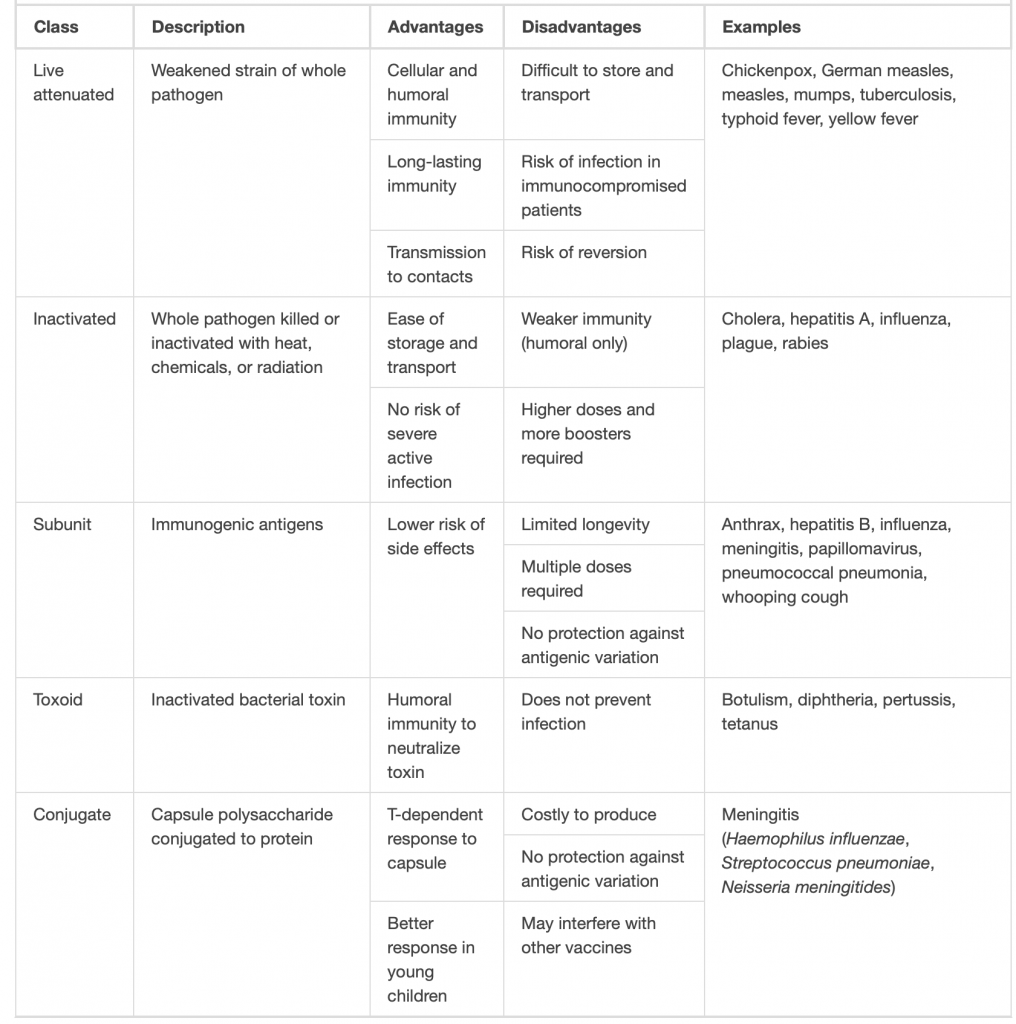
For a vaccine to provide protection against a disease, it must expose an individual to pathogen-specific antigens that will stimulate a protective adaptive immune response. By its very nature, this entails some risk. As with any pharmaceutical drug, vaccines have the potential to cause adverse effects. However, the ideal vaccine causes no severe adverse effects and poses no risk of contracting the disease that it is intended to prevent. Various types of vaccines have been developed with these goals in mind. These different classes of vaccines are described in the next section and summarized In the table.
Live Attenuated Vaccines
Live attenuated vaccines expose an individual to a weakened strain of a pathogen with the goal of establishing a subclinical infection that will activate the adaptive immune defenses. Pathogens are attenuated to decrease their virulence using methods such as genetic manipulation (to eliminate key virulence factors) or long-term culturing in an unnatural host or environment (to promote mutations and decrease virulence).
By establishing an active infection, live attenuated vaccines stimulate a more comprehensive immune response than some other types of vaccines. Live attenuated vaccines activate both cellular and humoral immunity and stimulate the development of memory for long-lasting immunity. In some cases, vaccination of one individual with a live attenuated pathogen can even lead to natural transmission of the attenuated pathogen to other individuals. This can cause the other individuals to also develop an active, subclinical infection that activates their adaptive immune defenses.
Disadvantages associated with live attenuated vaccines include the challenges associated with long-term storage and transport as well as the potential for a patient to develop signs and symptoms of disease during the active infection (particularly in immunocompromised patients). There is also a risk of the attenuated pathogen reverting back to full virulence.
Inactivated Vaccines
Inactivated vaccines contain whole pathogens that have been killed or inactivated with heat, chemicals, or radiation. For inactivated vaccines to be effective, the inactivation process must not affect the structure of key antigens on the pathogen.
Because the pathogen is killed or inactive, inactivated vaccines do not produce an active infection, and the resulting immune response is weaker and less comprehensive than that provoked by a live attenuated vaccine. Typically the response involves only humoral immunity, and the pathogen cannot be transmitted to other individuals. In addition, inactivated vaccines usually require higher doses and multiple boosters, possibly causing inflammatory reactions at the site of injection.
Despite these disadvantages, inactivated vaccines do have the advantages of long-term storage stability and ease of transport. Also, there is no risk of causing severe active infections. However, inactivated vaccines are not without their side effects.
Subunit Vaccines
Whereas live attenuated and inactive vaccines expose an individual to a weakened or dead pathogen, subunit vaccines only expose the patient to the key antigens of a pathogen—not whole cells or viruses. Subunit vaccines can be produced either by chemically degrading a pathogen and isolating its key antigens or by producing the antigens through genetic engineering. Because these vaccines contain only the essential antigens of a pathogen, the risk of side effects is relatively low.
Toxoid Vaccines
Like subunit vaccines, toxoid vaccines do not introduce a whole pathogen to the patient; they contain inactivated bacterial toxins, called toxoids. Toxoid vaccines are used to prevent diseases in which bacterial toxins play an important role in pathogenesis. These vaccines activate humoral immunity that neutralizes the toxins.
Conjugate Vaccines
A conjugate vaccine is a type of subunit vaccine that consists of a protein conjugated to a capsule polysaccharide. Conjugate vaccines have been developed to enhance the efficacy of subunit vaccines against pathogens that have protective polysaccharide capsules that help them evade phagocytosis, causing invasive infections that can lead to meningitis and other serious conditions. The subunit vaccines against these pathogens introduce T-independent capsular polysaccharide antigens that result in the production of antibodies that can opsonize the capsule and thus combat the infection; however, children under the age of two years do not respond effectively to these vaccines. Children do respond effectively when vaccinated with the conjugate vaccine, in which a protein with T-dependent antigens is conjugated to the capsule polysaccharide. The conjugated protein-polysaccharide antigen stimulates production of antibodies against both the protein and the capsule polysaccharide.
New vaccines. RNA based-vaccines
Conventional vaccines usually contain inactivated disease-causing organisms or proteins made by the pathogen (antigens), which work by mimicking the infectious agent. They stimulate the body’s immune response, so it is primed to respond more rapidly and effectively if exposed to the infectious agent in the future. RNA vaccines use a different approach that takes advantage of the process that cells use to make proteins: cells use DNA as the template to make messenger RNA (mRNA) molecules, which are then translated to build proteins. An RNA vaccine consists of an mRNA strand that codes for a disease-specific antigen. Once the mRNA strand in the vaccine is inside the body’s cells, the cells use the genetic information to produce the antigen. This antigen is then displayed on the cell surface, where it is recognised by the immune system.
- Unlike a normal vaccine, RNA vaccines work by introducing an mRNA sequence (the molecule which tells cells what to build) which is coded for a disease specific antigen, once produced within the body, the antigen is recognised by the immune system, preparing it to fight the real thing
- RNA vaccines are faster and cheaper to produce than traditional vaccines, and a RNA based vaccine is also safer for the patient, as they are not produced using infectious elements
- Production of RNA vaccines is laboratory based, and the process could be standardised and scaled, allowing quick responses to large outbreaks and epidemics
- Most current research is into RNA vaccines for infectious diseases and cancer, for which there are several early-stage clinical trials, there is also some early research into the potential of RNA vaccines for allergies
- There is still a lot of work to be done before mRNA vaccines can become standard treatments, in the meantime, we need a better understanding of their potential side effects, and more evidence of their long term efficacy